
Warning
Problem: The current name of your GitHub Pages repository ("Solution: Please consider renaming the repository to "
http://".
However, if the current repository name is intended, you can ignore this message by removing "{% include widgets/debug_repo_name.html %}" in index.html.
Action required
Problem: The current root path of this site is "baseurl ("_config.yml.
Solution: Please set the
baseurl in _config.yml to "Education
-
 University of PittsburghDepartment of Computational and Systems Biology
University of PittsburghDepartment of Computational and Systems Biology
Ph.D. CandidateAug. 2021 - present -
 Rowan UniversityB.S. in Chemical EngineeringSep. 2015 - May 2019
Rowan UniversityB.S. in Chemical EngineeringSep. 2015 - May 2019
Experience
-
 Generate BiomedicinesMachine Learning InternJune 2024 - Aug. 2024
Generate BiomedicinesMachine Learning InternJune 2024 - Aug. 2024 -
 Pfizer Inc.Associate ScientistJuly 2019 - July 2021
Pfizer Inc.Associate ScientistJuly 2019 - July 2021
Selected Publications (view all )
OMTRA: A Multi-Task Generative Model for Structure-Based Drug Design
Ian Dunn, Liv Toft, Tyler Katz, Juhi Gupta, Riya Shah, Ramith Hettiarachchi, David Ryan Koes
NeurIPS MLSB Workshop December 2025
Structure-based drug design (SBDD) focuses on designing small-molecule ligands that bind to specific protein pockets. We propose a unified approach in OMTRA, a multi-modal flow matching model that flexibly performs many tasks relevant to SBDD, including de novo design, molecular docking, and pharmacophore-conditioned generation. OMTRA obtains state-of-the-art performance on pocket-conditioned de novo design and docking. Additionally, we curate a dataset of 500M 3D molecular conformers, complementing protein-ligand data and expanding the chemical diversity available for training.
OMTRA: A Multi-Task Generative Model for Structure-Based Drug Design
Ian Dunn, Liv Toft, Tyler Katz, Juhi Gupta, Riya Shah, Ramith Hettiarachchi, David Ryan Koes
NeurIPS MLSB Workshop December 2025
Structure-based drug design (SBDD) focuses on designing small-molecule ligands that bind to specific protein pockets. We propose a unified approach in OMTRA, a multi-modal flow matching model that flexibly performs many tasks relevant to SBDD, including de novo design, molecular docking, and pharmacophore-conditioned generation. OMTRA obtains state-of-the-art performance on pocket-conditioned de novo design and docking. Additionally, we curate a dataset of 500M 3D molecular conformers, complementing protein-ligand data and expanding the chemical diversity available for training.
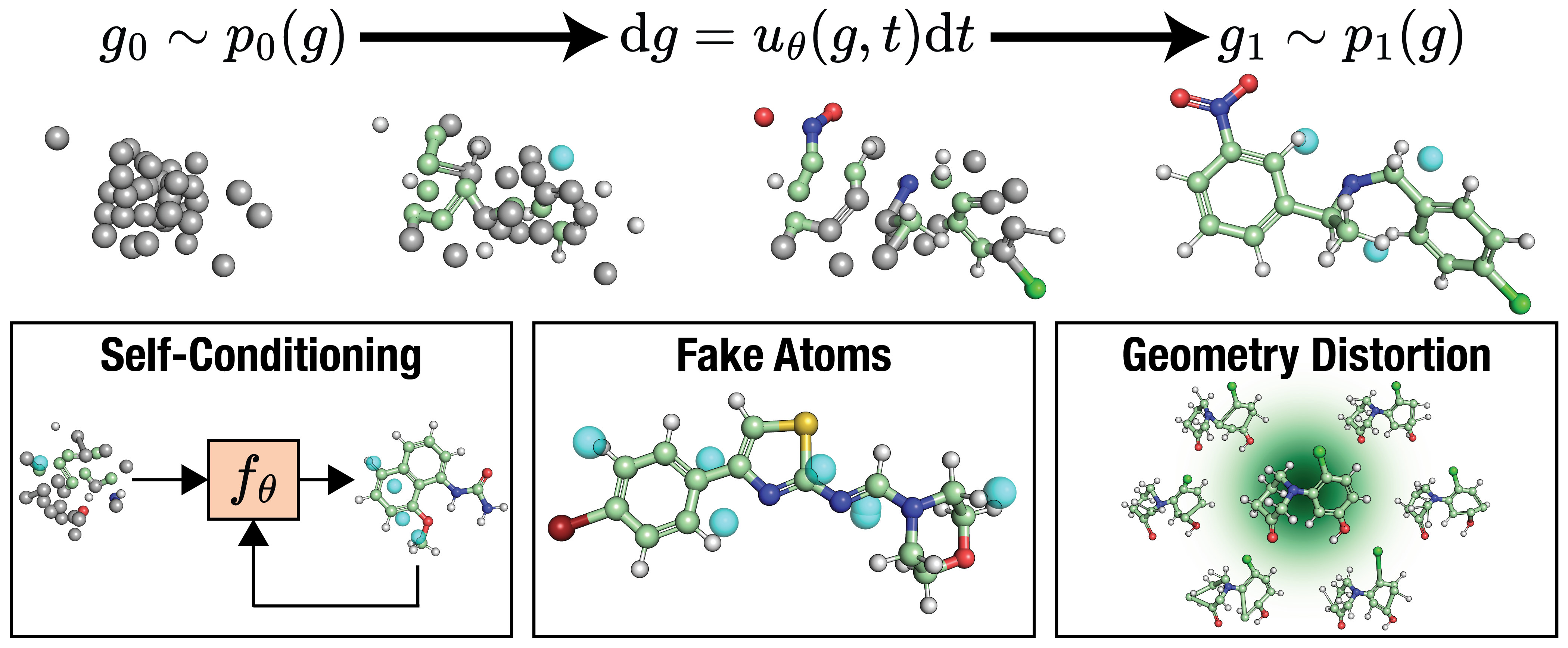
FlowMol3: Flow Matching for 3D De Novo Small-Molecule Generation
Ian Dunn, David Ryan Koes
Preprint September 2025
We present FlowMol3, a flow matching model that pushes the state of the art in unconditional 3D de novo small-molecule generation. FlowMol3 achieves nearly 100% molecular validity for drug-like molecules with explicit hydrogens, more accurately reproduces the functional group composition and geometry of its training data, and does so with an order of magnitude fewer learnable parameters than comparable methods.
FlowMol3: Flow Matching for 3D De Novo Small-Molecule Generation
Ian Dunn, David Ryan Koes
Preprint September 2025
We present FlowMol3, a flow matching model that pushes the state of the art in unconditional 3D de novo small-molecule generation. FlowMol3 achieves nearly 100% molecular validity for drug-like molecules with explicit hydrogens, more accurately reproduces the functional group composition and geometry of its training data, and does so with an order of magnitude fewer learnable parameters than comparable methods.
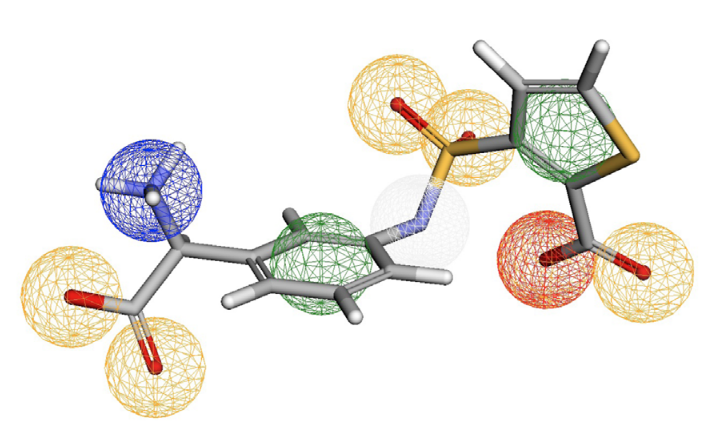
PharmacoForge: Pharmacophore Generation with Diffusion Models
Emma L. Flynn, Riya Shah, Ian Dunn, Rishal Aggarwal, David Ryan Koes
Frontiers in Bioinformatics September 2025
We present a machine learning approach to enhance structure-based drug discovery by generating three-dimensional pharmacophores using diffusion models. Rather than producing individual molecules, our method creates pharmacophore queries—spatial representations of protein-ligand interaction points—that can rapidly screen existing molecular databases for valid, commercially available compounds.
PharmacoForge: Pharmacophore Generation with Diffusion Models
Emma L. Flynn, Riya Shah, Ian Dunn, Rishal Aggarwal, David Ryan Koes
Frontiers in Bioinformatics September 2025
We present a machine learning approach to enhance structure-based drug discovery by generating three-dimensional pharmacophores using diffusion models. Rather than producing individual molecules, our method creates pharmacophore queries—spatial representations of protein-ligand interaction points—that can rapidly screen existing molecular databases for valid, commercially available compounds.
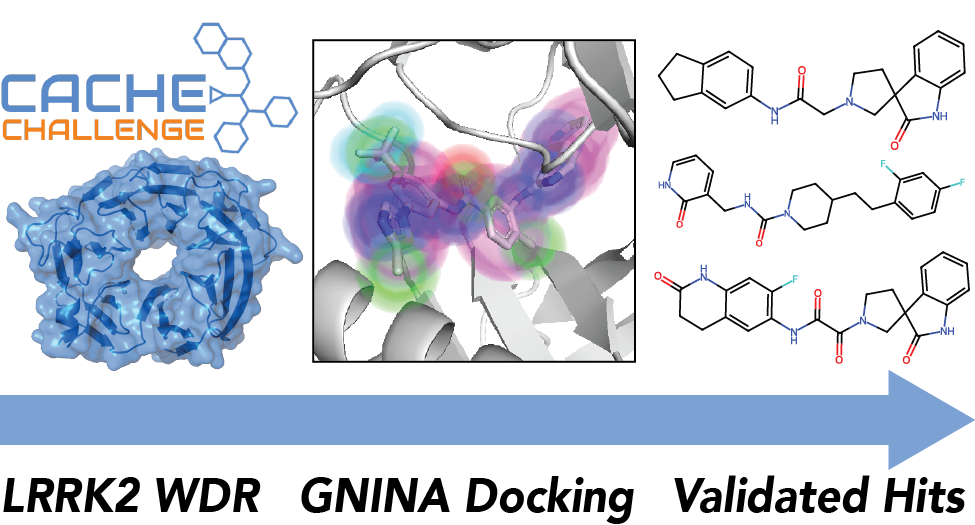
CACHE Challenge #1: Docking with GNINA is all you need
Ian Dunn, Somayeh Pirhadi, Yao Wang, Smmrithi Ravindran, Carter Concepcion, David Ryan Koes
Journal of Chemical Information and Modeling December 2024
We describe our winning submission to the first Critical Assessment of Computational Hit-Finding Experiments (CACHE) challenge. Our screening campaign was largely built around gnina, an open-source molecular docking program developed by the Koes lab that uses a deep-learning based scoring function. Our resulting best hit series tied for first place when evaluated by a panel of expert judges.
CACHE Challenge #1: Docking with GNINA is all you need
Ian Dunn, Somayeh Pirhadi, Yao Wang, Smmrithi Ravindran, Carter Concepcion, David Ryan Koes
Journal of Chemical Information and Modeling December 2024
We describe our winning submission to the first Critical Assessment of Computational Hit-Finding Experiments (CACHE) challenge. Our screening campaign was largely built around gnina, an open-source molecular docking program developed by the Koes lab that uses a deep-learning based scoring function. Our resulting best hit series tied for first place when evaluated by a panel of expert judges.